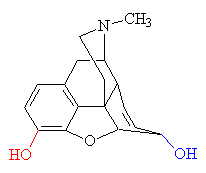
MORPHINE
Morphine is a benzylisoquinoline alkaloid
with two additional ring closures.
 Most of the
licit morphine produced is used to make codeine by methylation. It is also a precursor
for many drugs including heroin (diacetylmorphine), hydromorphone, and
oxymorphone. Replacement of the N-methyl group of morphine with an
N-phenylethyl group results in a product that is 18 times more powerful than
morphine in its opiate agonist potency. Combining this modification with the
replacement of the 6-hydroxyl with a 6-methylene produces a compound some 1,443
times more potent than morphine, stronger than the Bentley compounds such as
etorphine.
Most of the
licit morphine produced is used to make codeine by methylation. It is also a precursor
for many drugs including heroin (diacetylmorphine), hydromorphone, and
oxymorphone. Replacement of the N-methyl group of morphine with an
N-phenylethyl group results in a product that is 18 times more powerful than
morphine in its opiate agonist potency. Combining this modification with the
replacement of the 6-hydroxyl with a 6-methylene produces a compound some 1,443
times more potent than morphine, stronger than the Bentley compounds such as
etorphine.
The
structure-activity relationship of morphine has been extensively studied. The
structural formula of morphine was determined in 1925 and confirmed in 1952
when two methods of total synthesis were also published. As a result of the
extensive study and use of this molecule, more than 200 morphine derivatives
(also counting codeine and related drugs) have been developed since the last
quarter of the 19th Century. These drugs range from 25 per cent the strength of
codeine or a little over 2 per cent of the strength of morphine, to several
hundred times the strength of morphine to several powerful opioid antagoinsts including naloxone
(Narcan), naltrexone (Trexan), and nalorphine (Nalline) for human use and also
the amongst strongest antagonists known, such as diprenorphine (M5050), the
reversing agent in the Immobilon large animal tranquilliser dart kit; the
tranquilliser is another ultra-potent morphine derivative/structural analogue,
viz., etorphine (M99). Morphine-derived agonist-antagonist drugs have also been
developed. Elements of the morphine structure have been used to create completely
synthetic drugs such as the morphinan family (levorphanol, dextromethorphan and
others) and other groups which have many members with morphine-like qualities.
The modification of morphine and the aforementioned synthetics has also given
rise to non-narcotic drugs with other uses such as emetics, stimulants,
antitussives, anticholinergics, muscle relaxants, local anaesthetics, general
anaesthetics, and others.
Most
semi-synthetic opioids, both of the morphine and codeine subgroups, are created
by modifying one or more of the following:
- Halogenating or making other modifications at positions 1 and/or 2 on the morphine carbon skeleton.
- The methyl group which makes morphine into codeine can be removed or added back, or replaced with another functional group like ethyl and others to make codeine analogues of morphine-derived drugs and vice versa. Codeine analogues of morphine-based drugs often serve as prodrugs of the stronger drug, as in codeine & morphine, hydrocodone & hydromorphone, oxycodone & oxymorphone, nicocodeine & nicomorphine, dihydrocodeine and dihydromorphine, &c. &c.
- Saturating, opening, or other changes to the bond between positions 7 and 8, as well as adding, removing, or modifying functional groups to these positions; saturating, reducing, eliminating, or otherwise modifying the 7-8 bond and attaching a functional group at 14 yields hydromorphinol; the oxidation of the hydroxyl group to a carbonyl and changing the 7-8 bond to single from double changes codeine into oxycodone.
- Attachment, removal or modification of functional groups to positions 3 and/or 6 (dihydrocodeine and related, hydrocodone, nicomorphine); in the case of moving the methyl functional group from position 3 to 6, codeine becomes heterocodeine which is 72 times stronger, and therefore six times stronger than morphine
- Attachment of functional groups or other modification at position 14 (oxymorphone, oxycodone, naloxone)
- Modifications at positions 2, 4, 5 or 17, usually along with other changes to the molecule elsewhere on the morphine skeleton. Often this is done with drugs produced by catalytic reduction, hydrogenation, oxidation, or the like, producing strong derivatives of morphine and codeine.
Both
morphine and its hydrated form, C17H19NO3H2O, are sparingly soluble in water. In five liters of
water, only one gram of the hydrate will dissolve. For this reason,
pharmaceutical companies produce sulfate and hydrochloride salts of the drug,
both of which are over 300 times more water-soluble than their parent molecule.
Whereas the pH of a saturated morphine hydrate solution is 8.5, the salts
are acidic. Since they derive from a strong acid but weak base, they are both
at about pH = 5; as a consequence, the morphine salts are mixed with small
amounts of NaOH to make them suitable for injection.
A number of
salts of morphine are used, with the most common in current clinical use being
the hydrochloride, sulphate, tartrate, acetate, citrate; less commonly
methobromide, hydrobromide, hydroiodide, lactate, chloride, and bitartrate and
the others listed below. Morphine meconate is a major form of the alkaloid in
the poppy, as is morphine pectinate, nitrate and some others. Like codeine,
dihydrocodeine and other, especially older, opiates, morphine has been used as the salicylate
salt by some suppliers and can be easily compounded, imparting the therapeutic
advantage of both the opioid and the NSAID; multiple barbiturate salts of morphine were also used in
the past, as was/is morphine valerate, the salt of the acid being the active
principle of valerian. Calcium morphenate is the intermediate in
various latex and poppy-straw methods of morphine production. Morphine
ascorbate and other salts such as the tannate, citrate, and acetate, phosphate,
valerate and others may be present in poppy tea depending on the method of
preparation. Morphine valerate produced industrially was
one ingredient of a medication available for both oral and parenteral
administration popular many years ago in Europe and elsewhere called Trivalin
(not to be confused with the curremt, unrelated herbal preparation of the same
name) which also included the valerates of caffeine and cocaine, with a version
containing codeine valerate as a fourth ingredient being distributed under the
name Tetravalin.
Closely
related to morphine are the opioids morphine-N-oxide (genomorphine) which is a
pharmaceutical which is no longer in common use; and pseudomorphine, an
alkaloid which exists in opium, form as degradation products of morphine.
The salts
listed by the United States Drug Enforcement Administration for reporting
purposes, in addition to a few others, are as follows:
|
Forms of morphine, salts & chemical form to
freebase conversion ratios
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Production
A Hungarian
chemist, János Kabay, found and internationally patented a method to extract
morphine from poppy straw. In the opium poppy the alkaloids are bound to
meconic acid. The method is to extract from the crushed plant with diluted
sulfuric acid, which is a stronger acid than meconic acid, but not so strong to
react with alkaloid molecules. The extraction is performed in many steps (one
amount of crushed plant is at least six to ten times extracted, so practically
every alkaloid goes into the solution). From the solution obtained at the last
extraction step, the alkaloids are precipitated by either ammonium hydroxide or
sodium carbonate. The last step is purifying and separating morphine from other
opium alkaloids. Opium poppy contains at least 40 different alkaloids, but most
of them are of very low concentration. Morphine is the principal alkaloid in
raw opium and constitutes ~8-19% of opium by dry weight (depending on growing
conditions)
The
pharmacology of heroin and morphine is identical except the two acetyl groups
increase the lipid solubility of the heroin molecule, causing it to cross the
blood-brain barrier and enter the brain more
rapidly. Once in the brain, these acetyl groups are removed to yield morphine, which causes the subjective
effects of heroin. Thus, heroin may be thought of as a more rapidly acting form
of morphine..
Precursor to other opioids, in an underground and
illicit setting
Illicit
morphine is rarely produced from codeine found in over the counter cough and
pain medicines. This demethylation reaction is often performed using pyridine
and hydrochloric acid.
Another
source of illicit morphine comes from the extraction of morphine from extended
release morphine products, such as MS-Contin.
Morphine can be extracted from these products with simple extraction techniques
to yield a morphine solution that can be injected. Alternatively, the tablets
can be crushed and snorted, injected or swallowed, although this provides much
less euphoria although retaining some of the extended-release effect and the
extended-release property is why MS-Contin is used in some countries alongside
methadone, dihydrocodeine, buprenorphine, dihydroetorphine, piritramide,
levo-alpha-acetylmethadol (LAAM) and special 24-hour formulations of
hydromorphone for maintenance and detoxification of those physically dependent
on opioids.
Another
means of using or misusing morphine is to use chemical reactions to turn it
into heroin or another stronger opioid. Morphine can, using a technique
reported in New Zealand (where the initial precursor is codeine) and elsewhere
known as home-bake, be turned into what is usually a mixture of morphine, heroin, 3-monoacetylmorphine,
6-monoacetylmorphine, and codeine derivatives like acetylcodeine if the process
is using morphine made from demethylating codeine by mixing acetic anhydride or
acetyl chloride with the morphine and cooking it in an oven between 80 and 85°C
for several hours.
Since heroin
is one of a series of 3,6 diesters of morphine, it is possible to convert
morphine to nicomorphine (Vilan) using nicotinic anhydride, dipropanoylmorphine
with propionic anhydride, dibutanoylmorphine and disalicyloylmorphine with the
respective acid anhydrides. Glacial Acetic acid can be used to obtain a mixture
high in 6-monoacetylmorphine, nicotinic acid (Vitamin B3) in some form would be
precursor to 6-nicotinylmorphine, salicylic acid may yield the salicyoyl
analogue of 6-MAM, and so on.
Homebake or
other clandestinely-produced heroin produced from extended-release morphine tablets may be known as Blue Heroin
because of the blue colour of some of these tablets, even though the coloured
coating of the tablet is usually removed before processing, many strengths of
the tablets are not blue, bluish or a related colour like purple, and the final
product tends not to be blue. A writer of a 2006 description of producing
heroin from 100 mg as well as some 30 and 15 mg MS-Contin type
tablets coined the term Blue Heroin to distinguish his, her or their product
from New Zealand-style homebake as the process was shorter and began with
uncoated tablets which in the case of the 100 mg tablet was at or above 35
per cent morphine sulphate by weight, resulting in a final liquid injectable
which was brown-purple and quite potent. The drugs present in the final product
are limited to heroin, 6-monoacetylmorphine, 3-monoacetylmorphine, and morphine,
with the 6-MAM being just as or more sought than the heroin for reasons
elucidated in the Wikipedia heroin article.
The
clandestine conversion of morphine to ketones of the hydromorphone class or
other derivatives like dihydromorphine (Paramorfan), desomorphine (Permonid),
metopon &c. and codeine to hydrocodone (Dicodid), dihydrocodeine
(Paracodin) &c. is more involved, time consuming, requires lab equipment of
various types, and usually requires expensive catalysts and large amounts of
morphine at the outset and is less common but still has been discovered by
authorities in various ways during the last 20 years or so. Dihydromorphine can
be acetylated into another 3,6 morphine diester, namely
diacetyldihydromorphine (Paralaudin), and hydrocodone into the bacon.
Biosynthesis
The morphine is biosynthesized from the tetrahydroisoquinoline reticuline. It is converted into salutaridine, thebaine, and oripavine. The involucrated enzymes in this process are the salutaridine synthase, salutaridine:NADPH 7-oxidoreductase and the codeinone reductase
Morphine - the first alkaloid
Raw opium
contains some 20 alkaloid substances, one of which is morphine, in a typical yield of 10%.
Morphine was first isolated in 1805 by Friedrich Sertürner, an apothecary's
assistant in Paderborn, Germany, however its basic structure was not correctly
determined until 120 years later. In the 1800s morphine (known then as
laudanum) was a popular panacea and was available from grocers and markets. Later
on its addictive qualities led to its use and availability being severely
restricted.
Morphine is
isolated from opium in large quantities (over 1000 tons per year), although
most commercial opium is converted into codeine
by methylation. Morphine acts as an anesthetic without decreasing
consciousness, and it is one of the most powerful analgesics known. However it
also suppresses the respiratory system, and high doses can cause death by
respiratory failure. Its analgesic properties are related to the ability of the
molecule to fit into and block a specific receptor site on a nerve cell. This
eliminates the action of the pain receptor, preventing the pain signals
reaching the brain. This is similar to the way in which the body's natural
painkillers (endorphins and enkaphalins) work. The shape of the morphine
molecule is crucial to its ability to exactly fit into the active site on the
receptor - the 'lock-and-key' mechanism. The benzene group of the morphine
molecule fits snugly against a flat section of the receptor protein, whilst the
bent neighbouring group of carbon atoms fits into a nearby groove. This allows
the positively charged nitrogen atom to attach to a negatively-charged group on
the receptor, so locking the two molecules together.
Morphine blocks
deep aching pain, but has no effect on the fast pain that results from injury.
One side effect of the analgesic action is that the patient often gets a
feeling of detachment from the world, along with euphoria and sometimes
pleasure. It is this which makes morphine, and the other drugs related to it
(such as heroin), attractive to those who want to use drugs for 'recreational'
reasons. However all of these drugs are highly addictive, and the body rapidly
gets acclimatised to their use, such that increasingly large dosages are
required for the same effect.
|
|
|
|
|
Codeine - the
methoxy addition is shown in red.
|
|
|
Pethidine - the
parts of the molecule resembling the morphine structure are shown in red.
|
The
isolation of morphine was the beginning of alkaloid chemistry, which has
yielded many important medicinal substances. Although a satisfactory theory of
analgesic structure or action still eludes us, experimenters have developed a
number of synthetic analgesics related to morphine. The oldest is pethidine (also known as meperidine, Demerol
and about 40 other names). It was synthesised in 1939 by the German chemist Otto
Eisleb. It is less potent than morphine, but is still widely used for the
relief of post-operative pain. By replacing one of the -OH groups with a
methoxy group, morphine is converted into codeine, another powerful painkiller. When
mixed with paracetamol it goes by the trade name Tylenol. When ingested,
the -OCH3 group is converted back to -OH, regenerating morphine.



from the article, i have some problems:
BalasHapushow the process of making codeine by methylation using morphine? and why the method of methylation in used ? and whether it can use another method?
and how to modify the morphine that can be a non-narcotic drugs such as stimulants, vomiting, local anesthetics, and others?
The methylation can be carried out as follows. The commercial morphine base is suspended in pure toluene by stirring. The mixture is heated to bring about distillation, which starts between 90°C and 100°C and is continued until the boiling point reaches 111°C. The remaining suspension mixture, which is now perfectly free of Water, is kept protected from humidity and cooled down to 14°C under stirring. Then, an appropriate quantity of the titrated solution of the methylating agent is added, this quantity containing an excess of 5%, The morphine dissolves immediately, and the solution is then brought to boiling; which starts at about 80°C. The alcohol distils off with a part of the toluene and distillation is continued until the boiling point again reaches 111°C. The remaining solution contains now codeine and dimethylaniline, but does not contain any morphine. The codeine can be extracted in the usual manner with dilute mineral acid, whereby the aqueous phase is kept only slightly acid to litmus (pH 4 - 4.5) to prevent dimethylaniline from being extracted together with the codeine.
HapusThe abbreviated formulae M.OH and M.OCH3 will be used for morphine and codeine respectively. there are several compounds that when reacted with morphine can produce codeine. One of them, the methylation of morphine using methyl iodide as the methylating agent in alkaline solution:
M.OH + CH3I + KOH → M.OCH3 + KI + H2O
The use of methylation method is because we wanna replacing the -OH group in morphine with -OCH3 produces codeine. see the structure of morphine and codeine
"Another means of using or misusing morphine is to use chemical reactions to turn it into heroin or another stronger opioid." can you explain how do we change morphine by using a chemical reaction to be heroin? thank's
BalasHapushi elsa
Hapustransform morphine into heroin my knowledge in a way
diasetilmorfin synthetic compound which is a derivative of morphine. Compounds diasetilmorfin white crystalline, odorless, and bitter is a compound that became known quite dangerous.
but for its chemical reaction I have not found
Another friend might know?
thanks :)
i just wanna add liza's answer,,,
HapusHeroin is synthesized compounds diasetilmorfin which is a derivative of morphine.
Heroin or Diasetilmorfin obtained from acetylation of morphine in both phenolic and alcoholic hydroxyl groups (3,6-diasetilmorfin).for more details you can see the structure of morphine and heroin.
Acetylation (or in IUPAC nomenclature ethanoylation) describes a reaction that introduces acetyl functional group into a chemical compound. Deacetylation is the removal of acetyl groups.In addition, the process of introducing acetyl groups (resulting in Acetoxy group) into the compound, to be specific, replacement acetyl groups to active hydrogen atoms. Reactions involving the replacement of a hydrogen atom from the hydroxyl group with an acetyl group (CH 3
CO) yields a specific ester, acetate. Acetic anhydride is commonly used as an acetylating agent reacting with free hydroxyl groups. For example, it is used in the synthesis of Aspirin and heroin.
hi yulia
BalasHapusit seems there are so many problems
I might want a little to express my opinion to your problem that how to modify the morphine that can be a non-narcotic drugs
yulia,
I've read your article
the third paragraph of your article I read about the modification of morphine.
I think it's your problem answers
The modification of morphine and the aforementioned synthetics has also given rise to non-narcotic drugs with other uses such as emetics, stimulants, antitussives, anticholinergics, muscle relaxants, local anaesthetics, general anaesthetics, and others.
Most semi-synthetic opioids, both of the morphine and codeine subgroups, are created by modifying one or more of the following:
Halogenating or making other modifications at positions 1 and/or 2 on the morphine carbon skeleton.
The methyl group which makes morphine into codeine can be removed or added back, or replaced with another functional group like ethyl and others to make codeine analogues of morphine-derived drugs and vice versa. Codeine analogues of morphine-based drugs often serve as prodrugs of the stronger drug, as in codeine & morphine, hydrocodone & hydromorphone, oxycodone & oxymorphone, nicocodeine & nicomorphine, dihydrocodeine and dihydromorphine, &c. &c.
Saturating, opening, or other changes to the bond between positions 7 and 8, as well as adding, removing, or modifying functional groups to these positions; saturating, reducing, eliminating, or otherwise modifying the 7-8 bond and attaching a functional group at 14 yields hydromorphinol; the oxidation of the hydroxyl group to a carbonyl and changing the 7-8 bond to single from double changes codeine into oxycodone.
Attachment, removal or modification of functional groups to positions 3 and/or 6 (dihydrocodeine and related, hydrocodone, nicomorphine); in the case of moving the methyl functional group from position 3 to 6, codeine becomes heterocodeine which is 72 times stronger, and therefore six times stronger than morphine
Attachment of functional groups or other modification at position 14 (oxymorphone, oxycodone, naloxone)
Modifications at positions 2, 4, 5 or 17, usually along with other changes to the molecule elsewhere on the morphine skeleton. Often this is done with drugs produced by catalytic reduction, hydrogenation, oxidation, or the like, producing strong derivatives of morphine and codeine.
Both morphine and its hydrated form, C17H19NO3H2O, are sparingly soluble in water. In five liters of water, only one gram of the hydrate will dissolve. For this reason, pharmaceutical companies produce sulfate and hydrochloride salts of the drug, both of which are over 300 times more water-soluble than their parent molecule. Whereas the pH of a saturated morphine hydrate solution is 8.5, the salts are acidic. Since they derive from a strong acid but weak base, they are both at about pH = 5; as a consequence, the morphine salts are mixed with small amounts of NaOH to make them suitable for injection.
ok maybe that's my opinion
how do you or another friend????
thanks before :)
for Tiara and liza,,
BalasHapusthank's for your comment,,,
yuli i have question about your material in this blog.The last step is purifying and separating morphine from other opium alkaloids. Opium poppy contains at least 40 different alkaloids, but most of them are of very low concentration.
BalasHapusso my questions:
how to purify and separate the opium alkaloid morphine from the other?
and explain one of the 40 different alkaloids contained in opium poppy?
thanks
Substance in opium
HapusØ Codeine (INN) or 3-methylmorphine (a natural isomer of alcohol morphine, the other being the semi-synthetic 6-methylmorphine) is an opiate used for its analgesic, antitussive and antidiarrheal. Codeine is the second most predominant alkaloid in opium, up to 3 percent, it is far more common (Papaver bractreatum) Iranian poppy, and codeine is extracted from this species in some places although the below mentioned morphine methylation process is still much more common. It is considered the prototype of the weak to midrange opioids.
Ø Dihydrocodeine is used as an alternative or addition to codeine for these indications. It is available as the following salts, in rough order of frequency of use: bitartrate, phosphate, hydrochloride, tartrate, hydroiodide, methyliodide, hydrobromide, and sulfate. Salt to free base conversion factor of 0.67 is to bitartrate, 0.73 for phosphate, and 0.89 for the hydrochloride. Commonly available as tablets, solutions, potions, and other oral forms, dihydrocodeine is also available in several countries as a solution to the administration of subcutaneous and intra-muscular inside. As with codeine, intravenous administration should be avoided, because it can lead to anaphylaxis and dangerous pulmonary edema. In the past, dihydrocodeine suppositories are used, however, dihydrocodeine is available in suppository form on prescription.
Ø Xanax or dihydrocodeinone is a semi-synthetic opioid derived from either of two natural opiates - codeine and tebain. Xanax is orally active narcotic analgesic (pain reliever) and antitussive (cough suppressant). It is commonly available in tablet, capsule and syrup form, and is often compounded with other, non-opioid compounds generally less effective as paracetamol (also known as acetaminophen) or ibuprofen, often added to both reduce recreational use (as paracetamol can potentially cause fatal liver toxicity at high doses), and to provide possible synergies between xanax and analgesic effects of non-opioid compounds this. However, the effectiveness and safety of hydrocodone compound products versus hydrocodone-only products remains a highly contentious issue.
Ø Tebain (paramorphine) is an opiate alkaloid. A minor constituent of opium, tebain is chemically similar to both morphine and codeine, but stimulatory rather than depressant effects, causing convulsions similar to strychnine poisoning at higher doses [3] tebain this. Not used therapeutically, but can be converted into a variety of compounds including industry oxycodone, Oxymorphone, nalbuphine, naloxone, buprenorphine naltrexone and etorphine.
Ø Oxycodone is an opioid analgesic medication synthesized from opium-derived tebain. It was developed in 1916 in Germany, as one of several new semi-synthetic opioids in an attempt to improve on the existing opiates: morphine, diacetylmorphine (heroin), and codeine
Oxycodone oral medications are generally prescribed to relieve moderate to severe pain. Currently formulated as single ingredient products or products plus. Some common examples of compounding are oxycodone with acetaminophen / paracetamol or NSAIDs such as ibuprofen. These formulations are available as generics but are also made under various brand names.
Ø Ethylmorphine (also known as codethyline, dionine, and ethyl morphine) is an opiate narcotic analgesic (pain killer).
Ethylmorphine invented in Germany at Merck in 1884 and used as a weaker alternative to heroin for all indications. Chemistry, ethylmorphine is a morphine molecule-OC2H5 group substituted for the 3-OH aromatic. Therefore the closest chemical relative ethylmorphine is codeine, also known as methylmorphine. Ethylmorphine also has a hydromorphone analogue (ethyldihydromorphinone or 3-ethoxy-7 ,8-dihydro-Morphin-6-one), and dihydromorphine analogue known as ethyldihydromorphine, although none of them seem to be commercially distributed at the current time.
Yulia, Xanax is not dihydrocodeinone!!!
HapusYou said above: "Xanax or dihydrocodeinone is a semi-synthetic opioid derived from either of two natural opiates - codeine and tebain. Xanax is orally active narcotic analgesic (pain reliever) and antitussive (cough suppressant)."
how the method of formation of elements form the structure of morphine levorphanol
BalasHapusplease explain more details from your stated that heroin may be thought of as a more rapidly acting form of morphine..?
BalasHapusbecause heroin in the brain due to hydrolysis into monoasetilmorfin rapidly and eventually into morphine, then undergoes conjugation with amino glukuronik menajdi morphine 6-glukoronid the analgesic effect is stronger than morphine alone. Drug accumulation occurs in patients with renal failure.
HapusI was perusing about dihydrocodeine & codeine phosphate and I discovered your blog which is very fascinating. I do trust it's alright with you on the off chance that I stick them on my Pinterest board. A debt of gratitude is in order for sharing!
BalasHapusa little knowledge is dangerous, 98% of what has been stated here is gobbledygook, a potpourri of half truths with a lot of misunderstanding thrown in. The blind leading the blind. Just avoid drugs kids..
BalasHapus